- Nernst equation
- Nernst equation 'nernst- n an equation used to find the potential difference across a semipermeable membrane through which an ion diffuses between solutions where its concentration differs and that for a temperature of 37°C (98.6°F) gives a result in millivolts equal to plus or minus 61.5 times the logarithm of the ratio of the concentration of the ion on one side of the membrane to its concentration on the otherNernst Walther Hermann (1864-1941)German physical chemist. One of the founders of modern physical chemistry, Nernst was a professor of chemistry first at Göttingen and then at Berlin. His research topics included the thermodynamics of chemical equilibrium, the theory of galvanic cells, the mechanism of photochemistry, and the properties of vapors at high temperature and of solids at low temperature. In 1888 he published his derivation of the law of diffusion for electrolytes in the simple case when only two kinds of ions are present. The formulation is now known as the Nernst equation. In 1889 in a more extensive study he developed the fundamental relationship between electromotive force and ionic concentration. Perhaps his most notable contribution was his formulation of the third law of thermodynamics in 1906, for which he was awarded the Nobel Prize for Chemistry in 1920.
* * *
an equation for the voltage produced by an electrochemical reaction: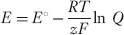
where E> is the voltage produced, E° is the standard reduction potential for the reaction, R> is the gas constant, T> the absolute temperature, z the number of electrons transferred in the reaction, F> Faraday constant, Q> the reaction quotient (q.v.), and ln the natural logarithm. The same formula gives the membrane potential produced by a concentration of a diffusible ion across a membrane; in this case E° is zero, z is the ionic charge, and Q> is the ratio of the concentrations on the two sides of the membrane.
Medical dictionary. 2011.
